Pharmacy
HEMANGEOL is the first and only FDA-Approved Treatment for Proliferating Infantile Hemangioma Requiring Systemic Therapy
How to fill for HEMANGEOL and apply benefits.
- Run Patient's benefits
- Whether covered or not covered, apply copay savings card as secondary to ensure patient maximum copay of $47
How to Dose HEMANGEOL
BID Dosing
- 3.4 mg/kg in 2 divided doses daily for 6 months
- Dosing Calculator for determining the appropriate dose for your patients.
week 1 – starting dose is 0.15mL/kg (0.6 mg/kg) twice daily
week 2 – increase dose to 0.3mL/kg (1.1 mg/kg) twice daily
week 3 – increase to a maintenance dose of 0.4mL/kg (1.7 mg/kg) twice daily - Readjust the dose periodically as the child’s weight increases.
- Administer twice daily doses at least 9 hours apart
- To reduce the risk of hypoglycemia, administer during or right after a feeding. Skip the dose if the child is not eating or is vomiting.
- Monitor heart rate and blood pressure for 2 hours after initiation or dose increases.
- Duration of treatment: 6 months
Efficacy – 60% of patients reached complete or nearly complete resolution of infantile hemangioma by 6 months versus 4% of patients on placebo
88% of patients on HEMANGEOL showed improvement after 5 weeks of treatment*
**Based on independent-investigator assessment of telangiectasis, color, textural change and distortion of anatomical landmarks or skin contours compared to baseline.
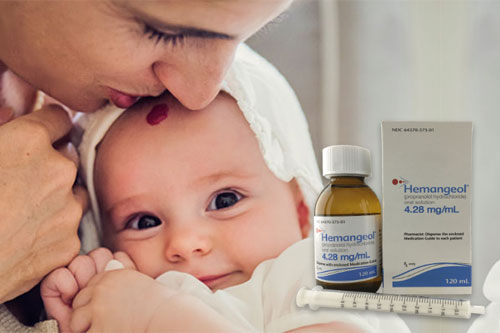
HEMANGEOL Dosing Chart
Our dosing chart lists weight, week duration in 1, 2, and 3 weeks, along with volume to be administered. Download the .PDF dosing chart below and keep for your records.
The HEMANGEOL Caregiver Guide
The Caregiver Guide brochure provides important information on how to recognize and to manage the following risks associated with the treatment with HEMANGEOL.
- Sugar (Hypoglycemia)
- Breathing Problems (Bronchospasm)
As with many drugs, HEMANGEOL may cause unwanted adverse effects.
These risks should be managed for the duration of treatment with HEMANGEOL for each child.
Alcohol Pediatric Guidelines
How much is too much when the pediatric minimal alcohol dose for toxicity is unknown?
HEMANGEOL Health & Safety
HEMANGEOL is a clear, colorless to slightly yellow oral-dosing solution supplied in a 120 mL amber glass bottle, with a child-resistant screw-cap. A specific oral syringe and syringe adapter are provided with each bottle. The oral dosing syringe measures HEMANGEOL according to volume in milliliters (mL).
- The most common adverse reactions to HEMANGEOL (occurring ≤10% of patients) were
- Sleep disorders
- Aggravated respiratory tract infections
- Diarrhea
- Vomiting
- Fewer than 2% of patients discontinued treatment due to adverse reactions
- The following adverse events were observed with incidence of less than 1%
- Cardiac disorders
- Decreased blood glucose
- Decreased heart beat
- Alopecia
- Urticaria
HEMANGEOL is:
- Alcohol-free
- Sugar-free
- Paraben-free
- No pediatric ethanol exposure

Important Safety Information for HEMANGEOL
Do not give HEMANGEOL to your child if your child:
- was born prematurely and has not reached the corrected age of 5 weeks
- weighs less than 4 ½ pounds
- is allergic to propranolol or any of the other ingredients in HEMANGEOL
- has asthma or a history of breathing problems
- has a heart problem, slow heart rate (less than 80 heart beats per minute), very low blood pressure
- is at risk for low blood sugar, for example is vomiting or unable to take feedings
- has high blood pressure caused by a tumor on the adrenal gland, called “pheochromocytoma”
Tell your doctor about all of your child’s medical conditions, all of the medicines your child takes, and all of the medicines that you take if you are breastfeeding your child.
HEMANGEOL can cause serious side effects, including:
Low blood sugar (hypoglycemia), especially if your child is not taking feedings, or is vomiting. HEMANGEOL may make it more difficult to recognize the signs and symptoms of low blood sugar in your child. To help reduce the risk of low blood sugar with HEMANGEOL, give HEMANGEOL during or shortly after feeding your child. Feed your child regularly during treatment. Tell your doctor if your child has a poor appetite. If your child is not taking feedings, due to an illness or vomiting, do not give HEMANGEOL until your child is taking feedings normally again.
If your child has any of the signs or symptoms of low blood sugar listed below during treatment with HEMANGEOL, stop giving your child HEMANGEOL and call your doctor or go to the nearest emergency room right away.
Signs or symptoms of low blood sugar include: pale, blue or purple skin color, sweating, irritability, crying for no apparent reason, irregular or fast heartbeat, poor feeding, low body temperature, unusual sleepiness, seizures, breathing stops for short periods of time, and loss of consciousness
If your child is conscious, give him/her a drink of a liquid containing sugar.
Other serious side effects can include:
- New or worsening slow heart rate (bradycardia) or low blood pressure (hypotension).
- Breathing problems or wheezing.
- Stroke. HEMANGEOL may increase the risk of stroke in certain children who have severe problems with the blood vessels in their brain, particularly if your child has a large hemangioma that affects the face or head.
Call your doctor or go to the nearest hospital emergency room if your child has:
- pale skin color, slow or uneven heartbeats, arms or legs feel cold, blue or purple skin color, or fainting.
- breathing problems or wheezing during treatment with HEMANGEOL.
The most common side effects include: sleep problems, worsening respiratory tract infections, diarrhea, and vomiting.
These are not all the possible side effects of HEMANGEOL. Call your doctor for medical advice about side effects.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see Full Prescribing Information and Medication Guide.